To understand complex movement in small molecules and macromolecules, it is easiest to begin with one of the simplest hydrocarbons, ethane (CH3CH3). Consider the two molecular models of ethane, shown below, in which grey indicates carbon and white indicate hydrogen. In the model on the left, the two hydrogen atoms highlighted in blue are on the same side of the molecule. In the model on the right, the hydrogen atoms highlighted in blue are on opposite sides of the molecule. Both models have the same atoms bonded together, but the spatial arrangements of the hydrogen atoms are clearly different.

These two representations of ethane are conformers of each other, because they differ by a simple bond rotation. We can see below that by rotating the model on the left about its C-C bond, we end up with the model on the right. Each time a bond rotation leads to a new spatial arrangement of atoms, we have a new conformation of the same molecule.
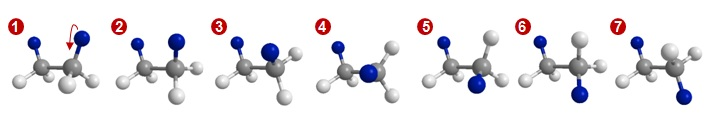
Snapshot of ethane molecule rotation across the C-C bond
σ-Bond rotations
We need to go back to what we’ve learned about hydrocarbon bonding to understand why free rotation occurs. Let’s focus on the central C-C bond in the molecule below. From Chapter 1.3, you learned that the bond between the two carbons is a sigma (σ) bond that results from the overlap of two sp3 hybridized orbitals. Recall that a σ-bond has cylindrical symmetry between the two carbon atoms. As you can see in the figure below, rotation of the carbon-carbon bond does not change the ability of the two carbon atoms to form a sigma bond.
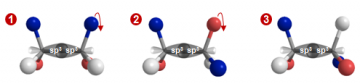
Rotation of a sigma bond