In a substitution reaction, one part of a molecule is substituted for a new part. For example, in the generic substitution reaction below, the X in the reactant is substituted for the A in the product.
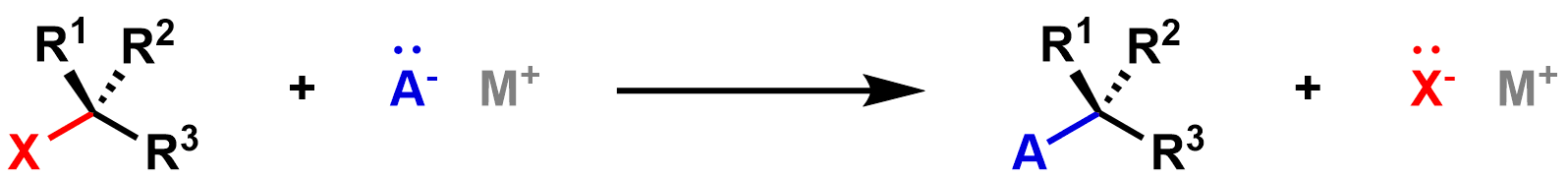
There are three key components in every substitution reaction: (1) the leaving group, (2) the nucleophile, and (3) the electrophile.
(1) Leaving group
The leaving group is the part of the molecule that leaves/is replaced. When it leaves, it takes the two electrons from the bond (shown in red above) with it, so it must be stable with these extra electrons. In the generic reaction above, the X is the leaving group. On the reactant side it is attached to the electrophile, and on the product side it has left as X-.
(2) Nucleophile
Nucleo- means nucleus, and -phile is latin for lover, so the nucleophile is a "nucleus lover" because it is attracted positive charges (such as the nucleus). The nucleophile has a lone pair of electrons available to share, usually with a partial or full negative charge. It shares this lone pair of electrons with the electrophile to form the new bond in the product. In the reaction above, the A- is the nucleophile, since it donates the electrons to form the new blue bond in the product.
(3) Electrophile
Electro- means electron, and -phile is latin for lover, so the electrophile is an "electron lover" because it is attracted to negative charges (such as electrons). Electrophiles tend to have full (or partial) positive charges, and are thus attracted to areas of high electron density (i.e. negative charges). In a substitution reaction, the electrophile accepts a lone pair of electrons from the nucleophile, which forms the new bond between the nucleophile and electrophile. In the reaction above, the electrophile is the black reactant on the left, since it accepts electrons from A-.
(4) Desired organic product
The desired organic product is the molecule that has a covalent bond between the electrophile and the nucleophile. Sometimes you will see only this product written in a substitution reaction, without the leaving group byproduct. It is also common to see the nucleophile written above the reaction arrow, rather than on the reactant side. An example using these two conventions is shown below:
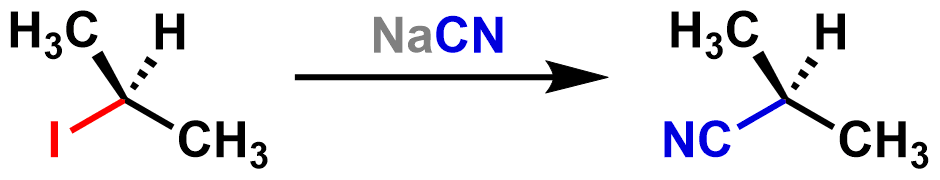
Chemists may write the reaction this way for efficiency or to emphasize the desired organic product. After all, a chemist carrying out a substitution reaction would use a purification technique to isolate only the desired organic product. When writing a full, balanced reaction, all products are included.
Note: the interactive components of this tutorial require html5 video, which is not supported by some mobile devices (e.g. iPhones). This tutorial is best viewed on a computer.
*Insert challenging question*
In a substitution reaction, one part of a molecule is substituted for a new part. For example, in the generic substitution reaction below, the X in the reactant is substituted for the A in the product.
In a substitution reaction, one part of a molecule is substituted for a new part. For example, in the generic substitution reaction below, the X in the reactant is substituted for the A in the product.
In a substitution reaction, one part of a molecule is substituted for a new part. For example, in the generic substitution reaction below, the X in the reactant is substituted for the A in the product.