Mechanism
Substitution reactions are subdivided into different types based on their mechanism. An SN2 reaction is a substitution reaction where the bond between the nucleophile and electrophile forms at the same time as the bond between the leaving group and electrophile breaks. This is called a concerted reaction, which means that all bonds are formed and broken in a single step. That is, the SN2 reaction occurs in a single elementary step, as shown in the diagram below:
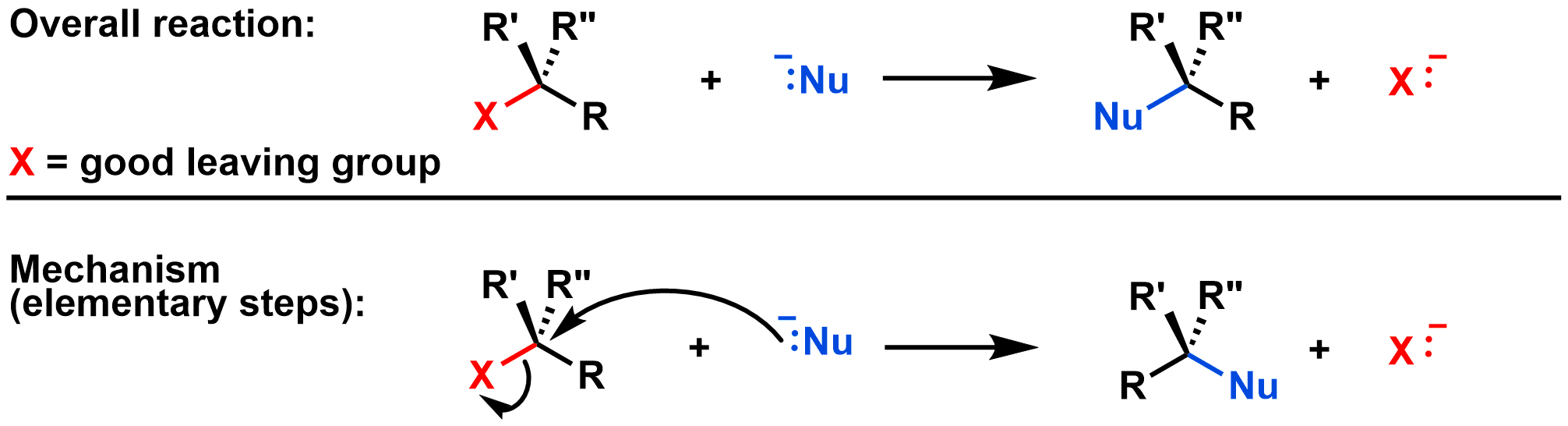
The video below shows a substitution reaction as it follows the SN2 mechanism.
Curved arrows: The first curved arrow shows the electrons from NC- (the nucleophile) moving towards the central carbon (on the electrophile) to form the new bond. The second curved arrow shows that the bond between the central C and Br breaks, and the electrons end up on the Br (the leaving group). Both curved arrows are drawn in the same step, since bond breaking and bond formation are simultaneous in the SN2 mechanism.
Transition state: The dotted lines in the video represent partially formed/broken bonds. As the bond between NC- and the central C forms, the bond between the central C and Br breaks. In the process the CH3 and two H's 'swing' from the left side to the right side. The transition state occurs when these groups are halfway through their 'swing' and the bonds are partially formed/broken. The transition state has a trigonal bipyramidal shape.
Backside attack:You may have noticed in the reaction above that the nucleophile forms a bond with the electrophile carbon that is directly bonded to the leaving group, and that it approaches the electrophile exactly opposite to the direction that the leaving group leaves. This approach is called "backside attack" and is generally true of all SN2 reactions. Two key factors help explain why the nucleophile attacks the carbon next to the leaving group via backside attack: the (1) orbital geometry and (2) electron distribution of the electrophile.
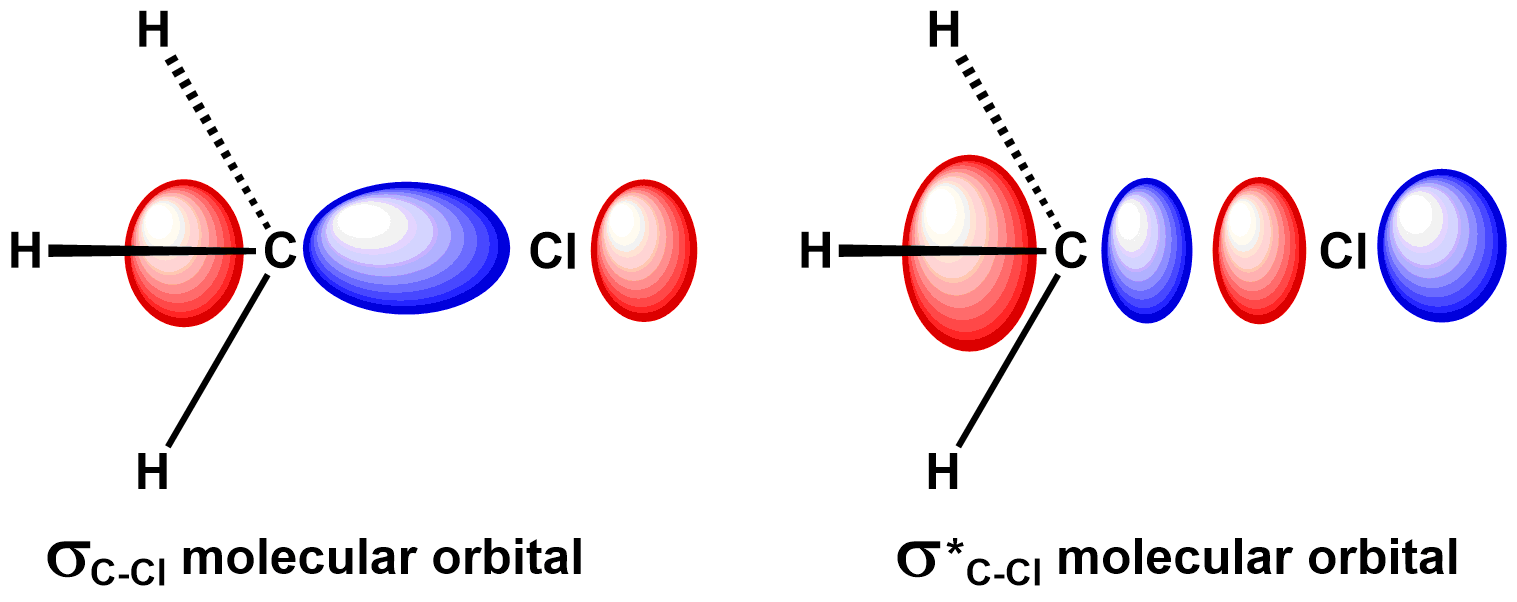
Let's first consider the orbital geometry. Consider the simplified bonding and antibonding molecular orbitals of the carbon-chlorine bond, below. During an SN2 reaction, the carbon-chlorine bond breaks when the nucleophile adds two electrons to the antibonding orbital. The antibonding orbital has a large lobes on chlorine and carbon, located on the far sides of the atoms relative to the carbon-chlorine bond. The nucleophile could break the chlorine-carbon bond by adding a pair of electrons to either of these large lobes. To understand why it attacks the lobe on carbon, let's consider the electron distribution in the electrophile.
The chlorine atom is more electronegative (3.16) than carbon (2.55), so the chlorine-carbon bond is polar, with the chlorine holding a larger share of the electrons. Thus, the chlorine atom bears a partial negative charge, and the carbon bears a partial positive charge, which we can visualize using either a dipole arrow or electrostatic potential map, both below.
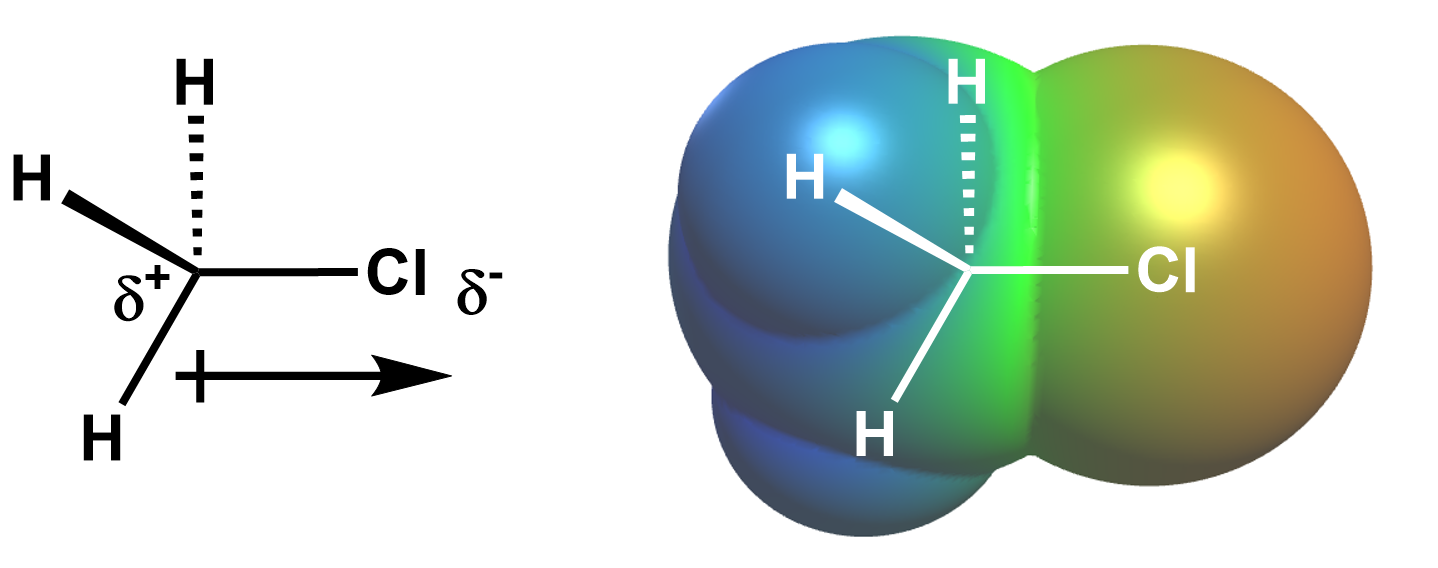
Since the nucleophile is negatively (or partially negatively) charged, it is attracted to the partially positively charged carbon next to the leaving group. Thus, in an SN2 reaction, the nucleophile attacks the electrophile carbon directly bonded to the leaving group via backside attack because the negatively charged nucleophile is attracted to this positively charged carbon, and an empty antibonding orbital capable of accepting electrons from the nucleophile points in this direction.
Kinetics
Since the SN2 reaction happens in a single step, it is an elementary reaction. With this information, you can determine the kinetics of SN2 reactions using your previous knowledge. Complete the activities below to see how your knowledge of kinetics applies to the SN2 mechanism. Note: The activities below require javascript. If they don't load on your device, try viewing this page on a computer.
Reaction coordinate diagram:
Rate law:
Reaction order:
Draw the mechanism of the SN2 reaction below:
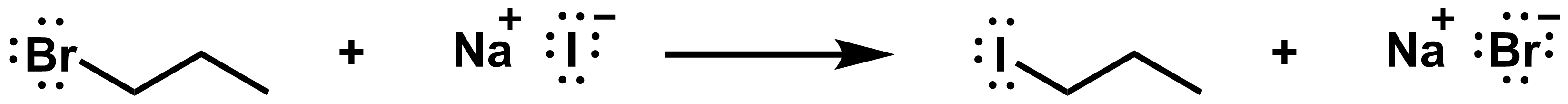
View solution:
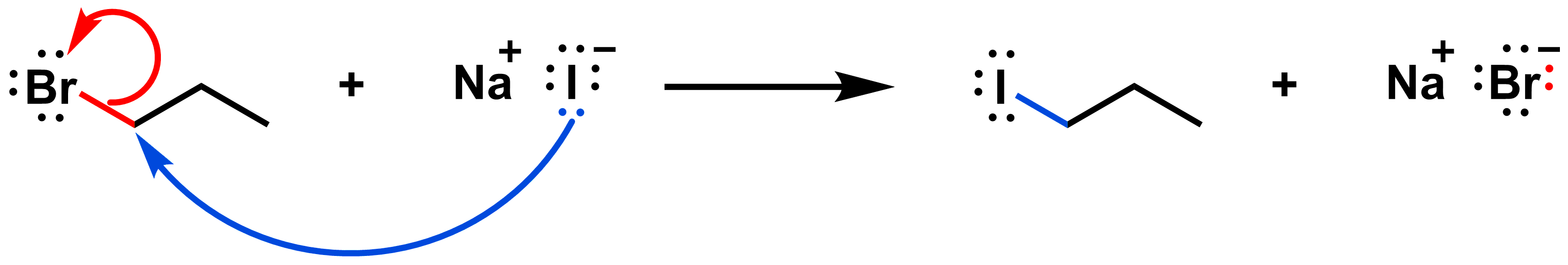
The red arrow shows how the red bond on the reactant side breaks. These electrons end up on the bromide on the product side. The blue arrow shows how the blue bond on the product side forms. A pair of electrons in iodide is shared with the reactive carbon.
Classify each molecule below as a (Z)-alkene or an (E)-alkene:
Classify each molecule below as a (Z)-alkene or an (E)-alkene:
Classify each molecule below as a (Z)-alkene or an (E)-alkene:
Classify each molecule below as a (Z)-alkene or an (E)-alkene: