Progress:
Electrophile:
In an SN2 reaction, the nucleophile approaches the electrophile from the side opposite to the leaving group. This means that the three other groups attached to the reactive carbon in the electrophile face towards the nucleophile as it approaches. If these three groups are small (e.g., all H's), then the nucleophile can approach easily, which can help the reaction proceed quickly. However, if these groups are all sterically bulky (e.g., all CH3 groups), then the nucleophile will have trouble getting close enough to the electrophile to react due to steric hindrance. For SN2 reactions, the smaller these three groups on the electrophile are, the faster the reaction proceeds.
Summary
The rest

Try rotating the space-filling models of bromomethane and 2-bromo-2-methylpropane below to see how much more accessible the reactive carbon (shown in grey, directly attached to the red bromine) is via backside attack in bromomethane compared to 2-bromo-2-methylpropane.
| bromomethane | 2-bromo-2-methylpropane |
We can classify electrophiles using the table below to help predict whether they will work well in an SN2 reaction.
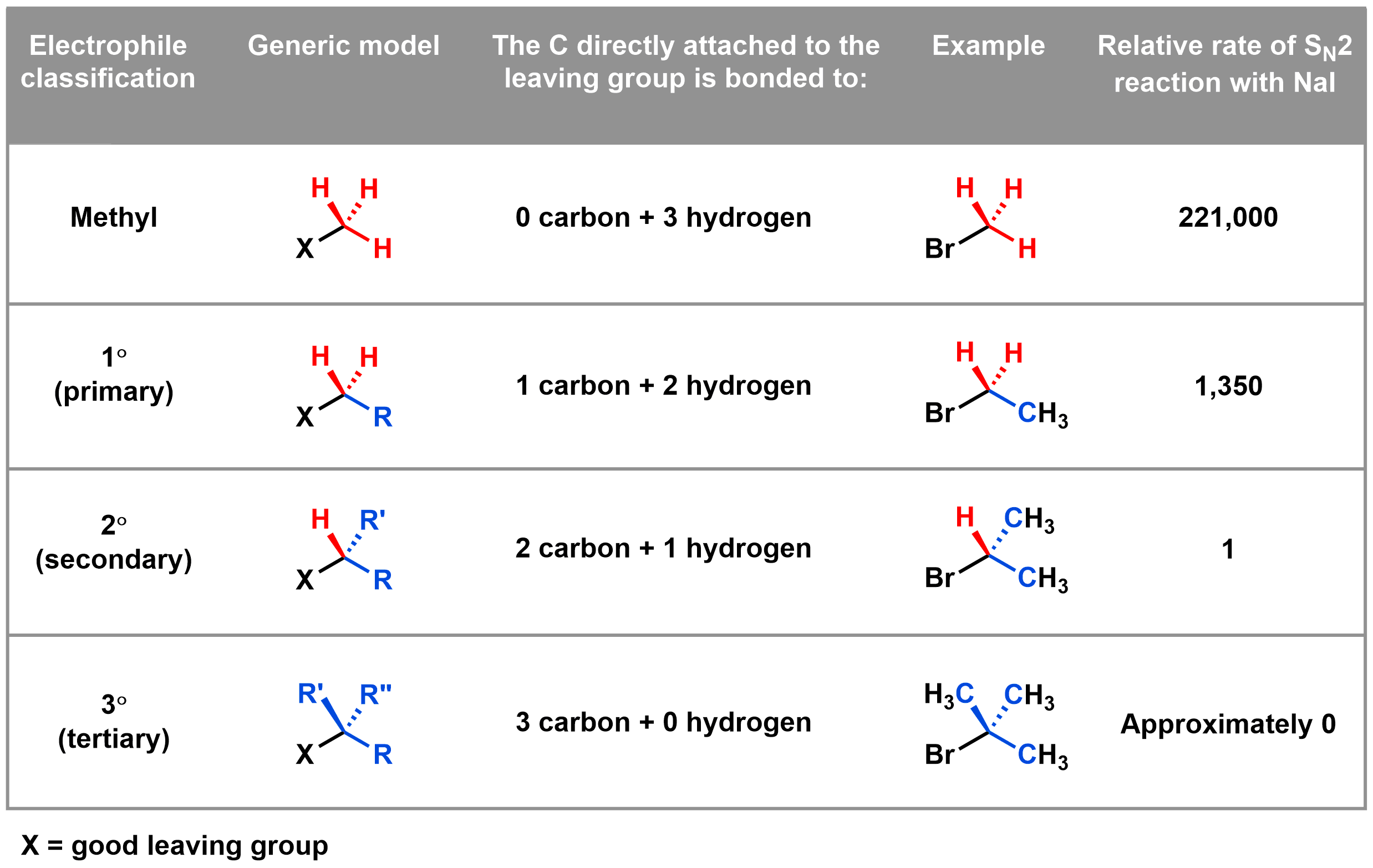
The last column in the above table gives us an idea of the relative rate of an SN2 reaction when using each of these nucleophiles. In a substitution reaction between the example electrophile (4th column) and NaI, the SN2 mechanism is 221,000 times faster with a methyl electrophile and 1,350 times faster with a 1° electrophile compared to a 2° electrophile. An SN2 reaction with a 3° electrophile does not proceed to an appreciable degree.
Nucleophile:
Nucleophiles donate pairs of electrons, so better nucleophiles more readily donate this pair of electrons. The more nucleophilic a molecule is, the faster it reacts in a substitution reaction. How good a nucleophile is depends on its charge, electronegativity, polarizability, and steric hindrance. You may learn about how the general trends below can sometimes differ, or even reverse, under different reaction conditions (e.g. using a different reaction medium) if you continue to study chemistry.
Charge: The higher the charge density, the more readily a nucleophile will donate a pair of electrons. Thus, negatively charged nucleophiles tend to be good, and neutral nucleophiles tend to be poor.

Electronegativity: When comparing the nucleophilicity of two molecules where the atoms donating the electrons are in the same row of the periodic table, the less electronegative atom holds onto its electrons less tightly and therefore is a better nucleophile.
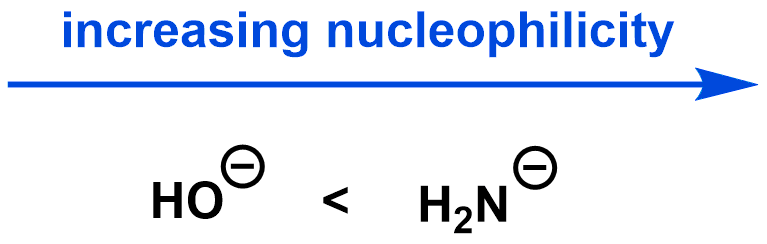
Polarizability: Nucleophilicity is dependent on polarizability, but the relationship is heavily dependent on the reaction medium (solvent), so is not covered here.
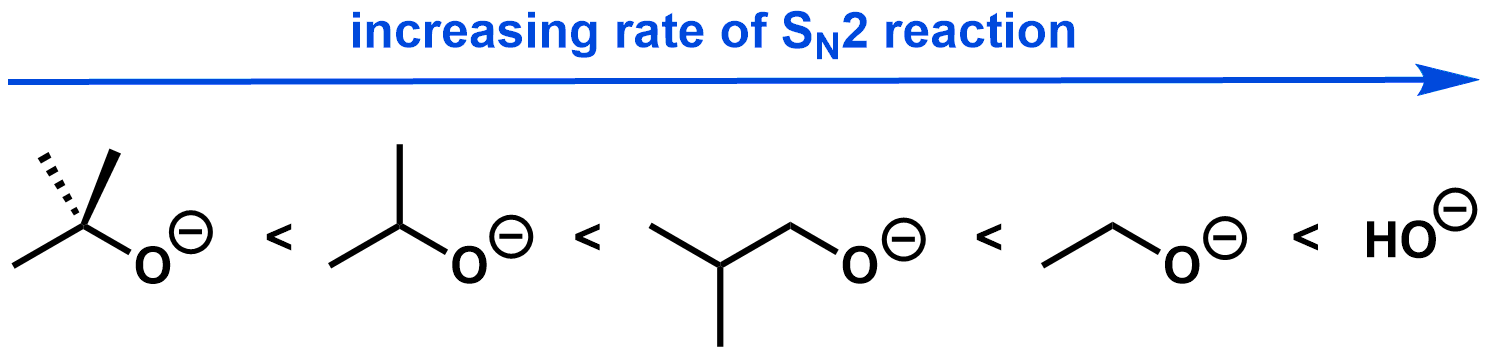
Steric hindrance: In an SN2 reaction, a highly branched nucleophile can result in steric hindrance during backside attack. Therefore, less bulky (i.e. less branched) nucleophiles react more quickly than more bulky nucleophiles.
Leaving group:
The leaving group ends up with an extra pair of electrons; the more stable the leaving group is with this extra pair of electrons, the better it is as a leaving group. Generally, leaving group ability is inversely related to basicity: the weaker a base is (i.e. the more stable it is), the better it is as a leaving group. You can use the exact same factors we used to assess the strength of a base (e.g. electronegativity, polarizability, resonance, and induction) to assess how good a leaving group is. If you come across leaving groups that do not follow this trend, it is likely that the reaction medium (solvent) is affecting this relationship.
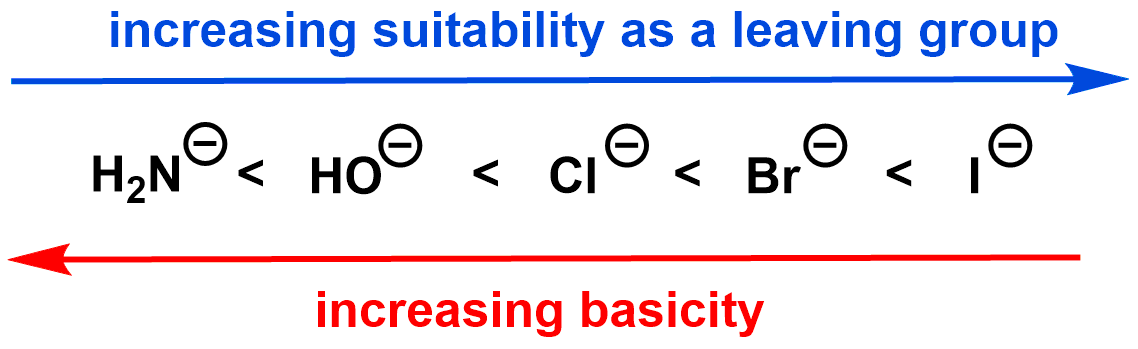
Neutral molecules such as H2O and NH3 make particularly good leaving groups.
Insert examples here.
Insert guided example here (in the form of an interactive video).
Insert easy question(s) here.
Insert medium question(s) here.
Insert challenging question(s) here.
Insert feedback form here.