Unlike single (sigma) bonds, double (sigma + pi) bonds are not free to rotate. In a carbon-carbon double bond, the pi bond forms between two adjacent p orbitals that are perpendicular to the plane of the bond. While the p-orbitals can maintain sufficient overlap to sustain the pi bond if the atoms vibrate back and forth slightly (see left video below), as soon as the rotation is significant the pi bond will break (see right video below).
As a result, some double bonds have two distinct isomers. For example, consider 2-butene shown below. In the 2-butene on the left, the H's are on the same side of the double bond. In the 2-butene on the right, the H's are on opposite sides of the double bond. Since we cannot simply rotate either molecule about its double bond to make the other without breaking the pi bond, these are two distinct isomers of 2-butene. In particular, these are a type of stereoisomers, which are isomers that have the same connectivity (i.e. bonds between atoms), but different 3D spatial arrangements.
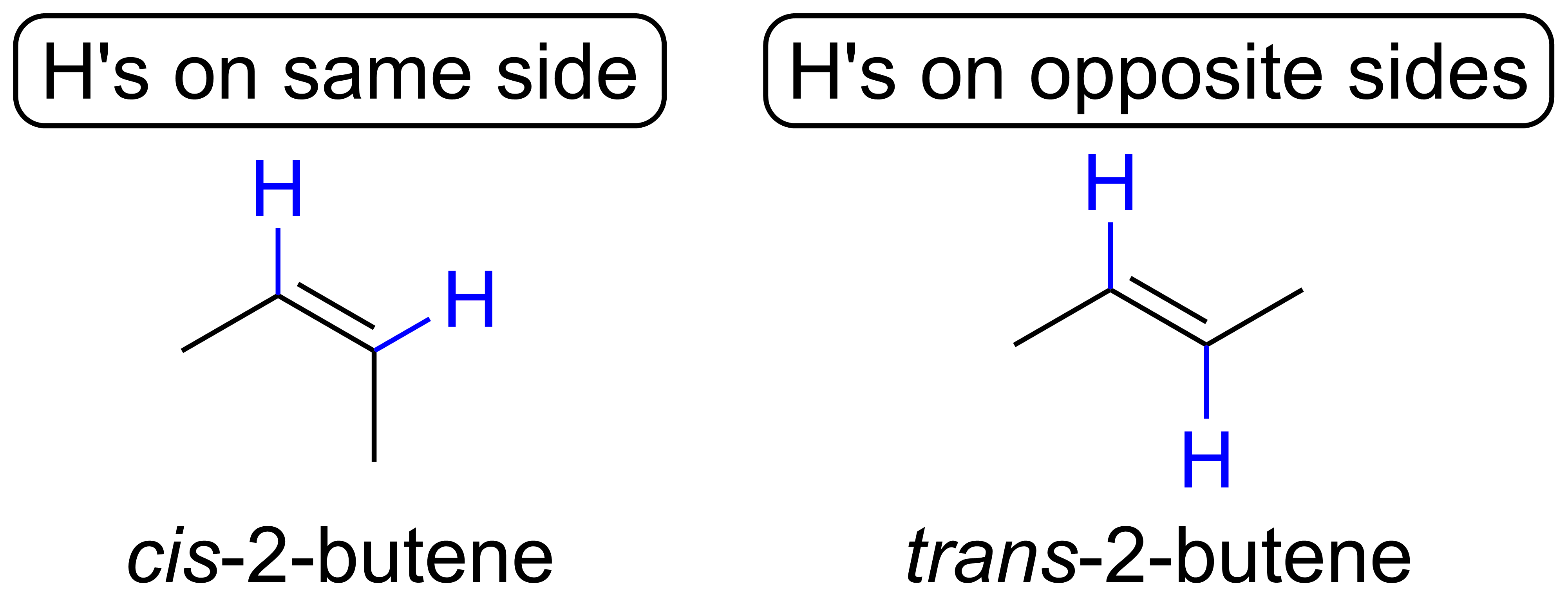
Two molecules are the same if you can:
1. Rotate one of the molecules about its sigma bonds to make it look like the other (i.e. they are conformers) and/or
2. Rotate one of the molecules in space to make it look like the other.
If the two molecules have the same connectivity, but no amount of rotation about sigma bonds or space will make them look the same, then they are stereoisomers, as shown in the examples below.

Try classifying each pair of molecules below as either stereoisomers or the same molecule: