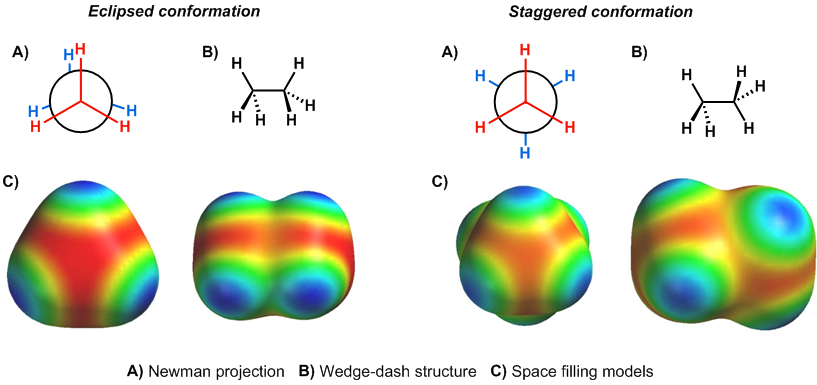
In section 1.1, we examined how there is free rotation around a sigma bond and how the rotation leads to different conformers. Lets examine the same type of rotation in ethane, but this time lets look at the different conformations using Newman Projections. For simplicity, we are going to keep one carbon fixed in place and just rotate the other, as is depicted in the diagram below. If we are to look at this rotation as a Newman Projection, we need to pick a direction to view down the central carbon-carbon bond. For this analysis, we will view from right to left (so the front carbon rotates), but the analysis would be the same looking from the opposite direction.
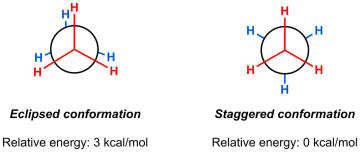
While there is free rotation about the central carbon-carbon bond, not all of the conformers are the same energy. The video below shows the rotation about ethane along with the energy at each point during the rotation. As you can see, there is a sinusoidal trend in the rotational energy diagram, with a relative minimum of 0 kcal/mol and a relative maximum of 3 kcal/mol.
From the video, you can see the relative maximum occurs when the hydrogens are aligned. This conformation is called an eclipsed conformation. When the hydrogens are perfectly spaced in the Newman projection, the energy minimum occurs, in what is called a staggered conformation. In ethane, there is a 3 kcal/mol difference in energy between these two conformations.
What is the reason for this energy difference? This was an active area of debate even within the past decade, which invoked different bonding models to rationalize the energy differences. The traditional theory that is still common in most textbooks is the eclipsed conformation has more significant repulsion between the C-H sigma bonds than the staggered conformation. Where does this repulsion come from? Recall from earlier chemistry courses that orbitals can only have two electrons because no two electrons can have the same atomic numbers (Pauli exclusion principle). In the staggered conformation, there is minimal overlap between the sigma bonds, while there is a fair amount of overlap in the eclipsed conformation. This overlap is difficult to see in line bond diagrams, but is much more apparent in space filling models when the electron clouds are included. This type of repulsion between two bonds is called torsional strain (or Pitzer strain).